HKBU research unveils an overlooked capability of microplastics in transforming adhered contaminants into a more harmful form

A research team led by Professor Kelvin Leung from the Department of Chemistry at Hong Kong Baptist University has revealed the complex and unique role of microplastics (MPs) in the accumulation and transformation of contaminants in natural environments. The alarming findings come shortly after their discovery of the formation of diverse complexes of pollutants on the MPs in various cocktails of environmental chemicals. This study provides the first evidence into how the co-occurrence of organic chemicals affects the sorption behaviors and oxidation states of MP-bound metals and ultimately their ecotoxicological effects.
The research results were recently published in Environmental Science & Technology Letters, a cutting-edge scientific journal.
Multi-contaminant interactions of MPs, heavy metals, and organic chemicals
Although numerous metals and organic chemicals have been found in environmental MP samples worldwide, there are no reports which have assessed the oxidation states of MP-bound metals with or without coexisting chemicals. This lack of information may lead to underestimating the ecological risks of MPs as carriers for trace metals and other contaminants in real environments.
In response to this knowledge gap, the research team examined the roles of organic chemicals in the sorptions and oxidation states of MP-bound metals and ultimately their ecotoxicological impacts. Polystyrene (PS), chromium (Cr) and benzophenone (BP)-type UV filters were used as the model MPs, metal, and organic chemicals, respectively.
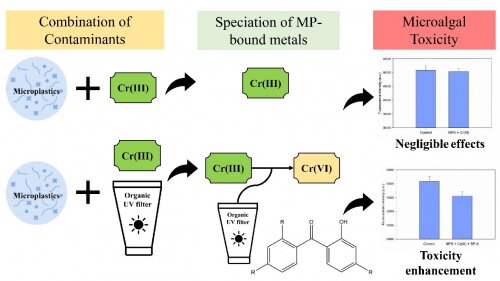
The results showed that the quantity of Cr sorption by PSMPs was apparently higher when BP-type UV filters were present. This enhanced affinity was attributed to the formation of Cr-UV filter complexes together with multilayer sorption on PSMPs’ surfaces. Subsequent chemical state measurements indicated that such interactions can alter the chemical forms of MP-bound metals as a higher Cr(VI) abundance was identified on the PSMP particles in the presence of a UV filter. Finally, the researchers also found that significant inhibitions of algae growth were observed when these Cr-UV filter complexes were present in the form of MP-bound chemicals. The team inferred that these Cr-UV filter complexes can be transformed into more toxic Cr(VI) species on the PSMPs’ surfaces and thus cause more harm to algae growth.
Our past and ongoing studies reveal that environmental contaminants are not just sticking to the MP particles, but also interacting with each other, ultimately altering the toxicity.
Professor Kelvin Leung
Department of Chemistry
Overlooked capability of microplastics in natural environments
Professor Leung said, “Previous research has well-documented that MPs are able to accumulate and concentrate other environmental contaminants on their surfaces, hence posing threats to aquatic life and human health. Our past and ongoing studies reveal that environmental contaminants are not just sticking to the MP particles, but also interacting with each other, ultimately altering the toxicity. For instance, the surprising results in this study unveil an overlooked capability of MPs–that is, the ability to transform adhered contaminants into a more harmful form. Our work could signal the tip of a hidden iceberg. More comprehensive assessments should be conducted as microplastics continue to accumulate in our air, water, and food.
Contact Our Researcher
DEPARTMENT OF CHEMISTRY
Related Publication
- Sorption Behavior, Speciation, and Toxicity of Microplastic-Bound Chromium in Multisolute Systems (2023) Environmental Science & Technology Letters Volume 10, Issue 1 https://pubs.acs.org/doi/10.1021/acs.estlett.2c00689
Previous News